Monitorizarea cardio-oncologică

Tratamentul oncologic (chimioterapia, radioterapia și intervențiile chirurgicale) a dus la îmbunătățirea semnificativă a supraviețuirii pacienților cu neoplazii, dar înprezența riscului de apariție a reacțiilor adverse.
În prezent, cele mai severe efecte adverse ale terapiei oncologice sunt reprezentate de complicațiile cardio-vasculare, potențial ireversibile și fatale. Astfel, incidența acestora poate ajunge până la 20-30%, devenind în prezent principala cauză de mortalitate a pacienților oncologici (depășind astfel mortalitatea determinată intrinsec de neoplazii).
În Statele Unite, există deja o nouă specialiate, cardio-oncologia, atât medicii cardiologi, cât și medicii oncologi, efectuând stagii de pregătire pentru a putea trata această afectare a inimii la pacienții care suferă de cancer.
Examinarea cardio-oncologică este esențială și reprezintă o necesitate pentru monitorizarea efectelor adverse potențiale ale tratamentului oncologic asupra inimii, pentru identificarea precoce a acestora și intervenția terapeutică rapidă în vederea reducerii morbidității și mortalității acestor pacienți.
Efecte adverse cardio-vasculare ale terapiei oncologice

- Cardiotoxicitatea sau disfuncția miocardică– afectarea funcției sistolice și/sau diastolice a inimii, definită că scăderea fracției de ejecție a ventricolului stâng sub 50% și cu mai mult de 10% din valoarea inițială, poate să apară acut (în primele ore de la administrarea tratamentului), subacut (în primele două săptămâni de la administrare tratament) sau cronic (precoce- în primul an de la terapie sau tardiv- după primul an). Această este determinată prin mecanism toxic direct al chimioterapicelor asupra mușchiului cardiac sau prin accelerarea ischemiei miocardice. Riscul de apariție și potențialul de evoluție către insuficiență cardiacă depinde de profilul de risc al pacienților, de asocierea radioterapiei mediastinale, precum și tipul de chimioterapice administrat.
Astfel, administrarea de Antracicline (doxorubicină, epirubicină, daunorubicină), folosite foarte frecvent în aproape toate neoplaziile solide, dar și limfoame și leucemii, determină afectare miocardică până la 48% din cazuri dependent de doză administrată cu potențial de evoluție către insuficiență cardiacă refractară și deces până la 16% din cazuri (mortalitatea acestei forme de insuficiență cardiacă este foarte mare – 60% la 2 ani). Administrarea de Anticorpi monoclonali (trastuzmab), folosit frecvent în cancerul de san, determina afectare miocardică la 4% din pacienți sau 27% din pacienți dacă se asociază la tratament și taxani (un alt tip de chimioterapic) sau radioterapie. Această formă de afectare miocardică este frecvent reversibilă la oprirea terapiei și nu depinde de doza administrată.
- Ischemia miocardică poate să apară rar după administrarea de taxani, 5-fluorouracil, Capecitabina, vincristina, rituximab sau sorafenib pentru cancerele mamare, colo-rectale, limfoame, leucemii sau cancerele hepatice sau tiroidiene sau doză mare de radioterapie.
- Agravarea sau inducerea afectării valvulare apar mai ales după asocierea radioterapiei mediastinale în doze mari.
- Tulburări de ritm și de conducere, pot să pară până la 16-36% și pot avea potențial letal.
- Hipertensiunea arterială poate să apară până la 47%, mai ales după administrarea bevacizumab sau sorafenib/sunitinib în cancerele colorectale, pulmonare sau renale.
- Afectarea coagulării sângelui, mai ales în cancerele metastatice după administrarea de Talidomida, cisplatin, bevacizumab, sunitinib, cu o incidența a trombembolismului venos de până la 20%.
- Risc de apariție sau agravare a bolii arteriale periferice sau cerebrale de până la 30%, mai ales în cazul radioterapiei mediastinale asociate cu risc crescut de accident vascular cerbral.
- Afectare pericardica sau apariția hipertensiunii pulmonare în cazul administrării ciclofosfamidei, bleomicinei sau asocierii de radioterapie.
Diagnosticul complicațiilor cardiovasculare induse de terapia oncologică
- Electrocardiogramă pentru identificarea tulburărilor de ritm și de conducere.
- Ecocardiografia convențională (2D și Doppler) pentru evaluarea funcției sistolice și diastolice și a structurii cavităților cardiace, a vâlvelor și pericardului. Calculul fracției de ejecție a ventricolului stâng se folosește pentru definiția cardiotoxicității și este un parametru ce trebuie măsurat obligatoriu pentru monitorizarea terapiei oncologice.
- Ecocardiografia 3D pentru calculul fractiei de ejectie a ventriculului stâng este recomndată de ghiduri pentru monitorizarea terapiei oncologice, atunci când este disponibilă, având avantaje față de ecografia convențională.
- Ecocardiografia prin Doppler tisular
- Ecocardiografia de deformare miocardică (Speckle Tracking eco) cu măsurarea parametrilor de deformare miocardică – strain, strain rate, torsiune de ventricul stâng, dintre care măsurarea periodică a strain-ului longitudinal de ventricul stâng este recomandată de ghiduri pentru monitorizarea terapiei oncologice. Are avantajul detecției precoce a afectării miocardice indusă de chimio sau radioterapie (înaintea afectării fracției de ejecție a ventricului stâng).
Alte investigații ce pot fi făcute pacienților oncologici, în funcție de semnele și simptomele de boală sau de suspiciunea de boală, la indicația medicului cardiolog pot fi: ECG de efort, monitorizare Holter TA sau ECG, ecocardiografia de contrast sau de stres, imagistică prin rezonanță magnetică cardiacă (Cardio-RM) sau tomografie computerizată cardiacă, precum și investigații de laborator (specfic troponine hs).
Algoritm de monitorizare a pacienților oncologici
(recomandat de ghidurile din 2014 și 2016, cu precizarea că poate fi modificat de către medicul cardiolog, individualizat, în funcție de profilul de risc al pacientului, de terapia oncologică folosită, precum și de apariția semnelor sau simptomelor de apariție a bolii cardiovasculare).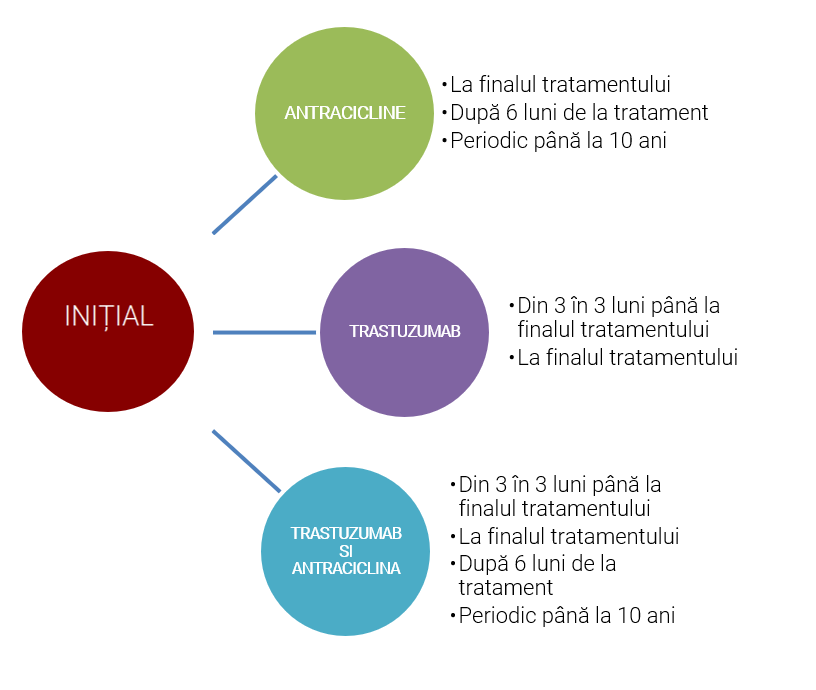
- La pacienții oncologici fără semne/simptome de boală cardiovascularăinițial sau în timpul terapiei oncologice : ECG, ecocardiografie convențional /3D (unde este disponibil), ecografie prin Speckle tracking (strain longitudinal), eventual troponină hs.
- La pacienții tratați și cu radioterapie mediastinală – evaluare prin ecocardiografie convențională/3D, ecocardiografie prin speckle tracking și după 10 ani de la radioterapie și apoi din 5 în 5 ani (risc de afectare valvulară sau pericardică) sau ecografie Doppler de artere cervicale sau de membre superioare din 5 in 5 ani (risc de stenoze de artere cervicale sau de membre superioare).
- Individualizat, oricând, la indicația medicului cardiolog, dacă evoluția pacientului o cere.